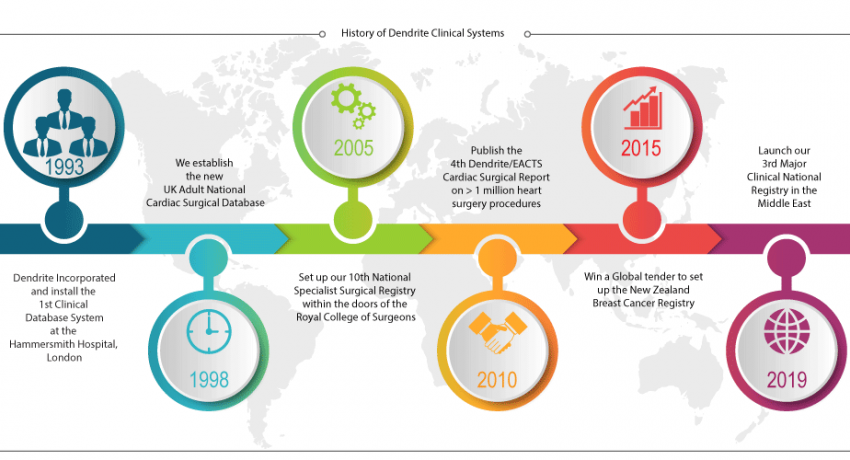
History
Dendrite Clinical Systems was established in 1993, by co-directors Dr Peter Walton, Keith Price and Neal McCann, and over the last 26 years the company has become one of the leading suppliers of clinical software to hospitals and institutions in the world. Since its inception, the company has maintained a belief that clinical data collection and analysis is crucial in achieving effective clinical audit and ultimately, improving patient outcomes.
Starting from an office in central London, Dendrite has evolved from a specialist supplier of clinical databases and analysis software, to providing consultancy and publishing services for the international healthcare sector. The company now has two offices in the UK, agents across four continents and has expanded its global user base across hundreds of hospitals, with over 170 national and international registries in more than 40 countries.
Dendrite has continued to develop innovative clinical software solutions, which have been utilised in many different clinical scenarios, fulfilling the company's ethos of providing the tools for effective clinical governance.
 Sapphire Medical Clinics and Dendrite Clinical Systems have launched the UK Medical Cannabis Registry, an initiative that is designed to rapidly expand the evidence base for medical cannabis in the UK and decrease the cost of access for patients. The launch will collect and analyse clinical information on patients taking medical cannabis treatments for all recognised eligible conditions.
Sapphire Medical Clinics and Dendrite Clinical Systems have launched the UK Medical Cannabis Registry, an initiative that is designed to rapidly expand the evidence base for medical cannabis in the UK and decrease the cost of access for patients. The launch will collect and analyse clinical information on patients taking medical cannabis treatments for all recognised eligible conditions. “I am very pleased to report that demand for the Dendrite Web-Registry platform has reached an all-time high” Dr Peter Walton, Managing Director of Dendrite Clinical System. “We’re receiving new orders for new national and international registries every few days now – for surgical registries, medical registries, rare disease registries, medical device registries and Coronavirus Registries (for a range of Community, National, International and Global Covid-19 Registries).”
“I am very pleased to report that demand for the Dendrite Web-Registry platform has reached an all-time high” Dr Peter Walton, Managing Director of Dendrite Clinical System. “We’re receiving new orders for new national and international registries every few days now – for surgical registries, medical registries, rare disease registries, medical device registries and Coronavirus Registries (for a range of Community, National, International and Global Covid-19 Registries).” Sapphire Medical Clinics and Dendrite Clinical Systems have established the UK’s first national UK’s first comprehensive medical cannabis patient registry - UK Medical Cannabis Registry. The UK Medical Cannabis Registry will be the first of its kind covering all conditions for which there is evidence of clinical efficacy of medical cannabis. The formation of the registry means that real world evidence, cited as a key barrier to wider patient prescriptions by NHS England, will now be available on request to the medical community for analysis.
Sapphire Medical Clinics and Dendrite Clinical Systems have established the UK’s first national UK’s first comprehensive medical cannabis patient registry - UK Medical Cannabis Registry. The UK Medical Cannabis Registry will be the first of its kind covering all conditions for which there is evidence of clinical efficacy of medical cannabis. The formation of the registry means that real world evidence, cited as a key barrier to wider patient prescriptions by NHS England, will now be available on request to the medical community for analysis. The British Association of Plastic, Reconstructive and Aesthetic Surgeons (BAPRAS), in conjunction with Dendrite Clinical Systems, is delighted to announce the release of the First UK National Flap Registry (UKNFR) Report. The UKNFR, which was launched in August 2015, collects information on all major free and pedicled flap operations carried out in the UK.
The British Association of Plastic, Reconstructive and Aesthetic Surgeons (BAPRAS), in conjunction with Dendrite Clinical Systems, is delighted to announce the release of the First UK National Flap Registry (UKNFR) Report. The UKNFR, which was launched in August 2015, collects information on all major free and pedicled flap operations carried out in the UK. Dendrite Clinical Systems, the publisher of Bariatric News, has announced the publication of the Bariatric News Industry and Product Guide 2019 – a comprehensive A-Z guide to companies, products and services within the bariatric and metabolic specialty.
Dendrite Clinical Systems, the publisher of Bariatric News, has announced the publication of the Bariatric News Industry and Product Guide 2019 – a comprehensive A-Z guide to companies, products and services within the bariatric and metabolic specialty.  Dendrite Clinical Systems, the publisher of Bariatric News, is pleased to announce issue 39 of the newspaper is now available to view/download. The newspaper reports on research, technology, events and policy in the bariatric specialty, the latest clinical studies, policy changes and product news, the latest meetings and events, interviews prominent bariatric experts, and host debates between specialists on controversial topics.
Dendrite Clinical Systems, the publisher of Bariatric News, is pleased to announce issue 39 of the newspaper is now available to view/download. The newspaper reports on research, technology, events and policy in the bariatric specialty, the latest clinical studies, policy changes and product news, the latest meetings and events, interviews prominent bariatric experts, and host debates between specialists on controversial topics.


